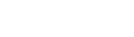QORATM stool management kit: The most advanced standard of Fecal Incontinence Management
Fecal incontinence (FI) is the inability to control bowel movements, resulting in the involuntary excretion of liquid, mucous, or solid stool from the rectum. Ranging in severity from minor soiling to a complete loss of bowel control, FI is a psychologically distressing condition that significantly affects quality of life. Although estimates of prevalence vary, recent studies from regions where data is available reflect an 8-15 percent incidence in adults in the general community, with the highest incidence linked to advanced age. FI is managed primarily with absorbent pads and adult diapers, which are time and labour-intensive as well as unpleasant for caregivers to manage.
At a Fellowship at Stanford-India Biodesign (SIB) program, we got the opportunity to spend more than 2000 hours in hospitals in India and US, including rural and community level hospitals to understand the problem at hand. Funded by the Department of Biotechnology, Ministry of Science and Technology, and Government of India, the SIB Program is centered in New Delhi and administered as a collaboration between Stanford University, the Indian Institute of Technology Delhi, the All India Institute of Medical Sciences (AIIMS) and Queensland University of Technology. Through the opportunity provided by the SIB program, we identified FI as a latent clinical need with few good treatment options.
After carefully evaluating the needs of the many stakeholders in FI care, we founded Consure Medical and set out to develop a solution that would solve the problems inherent in existing FI treatments and be simple enough for a motivated family member to use.
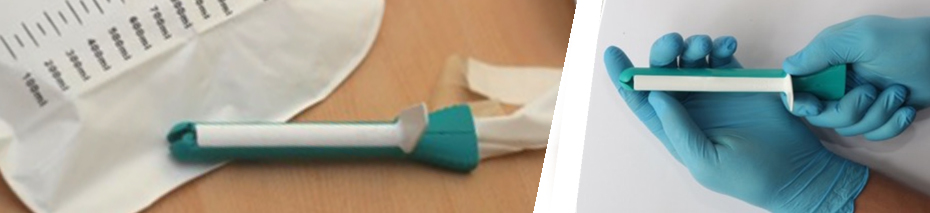
In collaboration with the top clinicians from Stanford and AIIMS, Consure Medical developed an indwelling device similar to a short-term implant. Although developed specifically for the Indian market, the team quickly realized that the device had global potential and could deliver significant value proposition to long-term and acute care settings. Not only was the Consure device more appealing to nurses than other drainage systems because of its ease of insertion, but also it could reduce the costs of caring for FI patients because it could be placed without a doctor, imaging, or pre-insertion exam. In addition to ease of use, drainage systems are preferred devices for clinicians on patients with sacral pressure ulcers and risk of nosocomial infections. Another source of efficiency was the reduction in time and labor associated with changing and cleaning patients who otherwise relied on absorbent solutions. With support from DBT which helped with providing initial grant for prototyping and testing, we developed over 150 ideas of prototypes and series of successful in-patient studies, and launched ‘QoraTM SMK’, an advanced standard of FI management in bed-ridden patients.
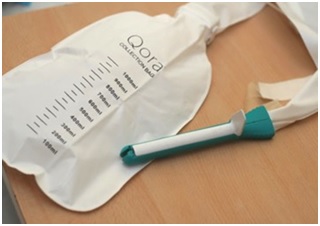
It is a great matter of pride for us, at Consure Medical, that QoraTM is the world’s first bowel management kit that expands indications for use from existing closed system solutions. Prospective cohort safety and efficacy studies at tertiary care hospitals have revealed improved clinical outcomes for patients and reduced time required to manage a patient’s incontinence. Relying on a proprietary diverter, the QoraTM suite of solutions have presented one of the most advanced and patient-friendly indwelling fecal drainage devices that increase the addressable market three-fold to reach patients in long-term care and palliative care facilities, reduce healthcare costs by avoiding complications due to exposure to fecal exudate, and provide a more effective and dignified standard of care. The devices are designed to cover the spectrum of clinical needs observed in institutionalized, fecal incontinent patients and has recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for expanded use period for up to 29 days.